Identification of Bioactive Compounds and Medicinal Chemistry:
Synthesis of new carbazole derivatives as anticancer agents:
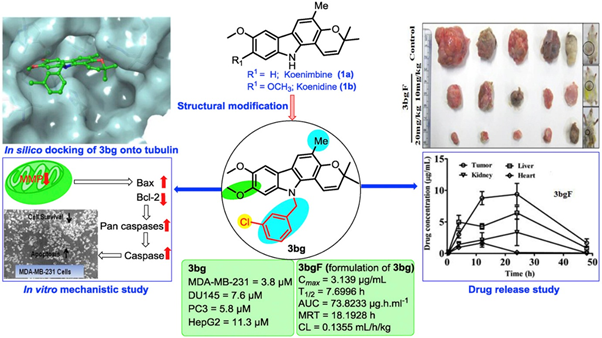
In this study we demonstrated that the pyranoccarbazole derivative 3bg and its formulation 3bgF significantly inhibited tumor growth in LA-7-induced syngeneic mammary tumors in SD rats with both 10 and 20 mg/kg body weight at the oral administration for 30 consecutive days. The findings comprehensively establish that pyranocarbazole derivatives has significant prospect as anticancer lead with potent in vitro and in vivo activity. (Eur. J. Med. Chem. 2019, 167, 226-244)
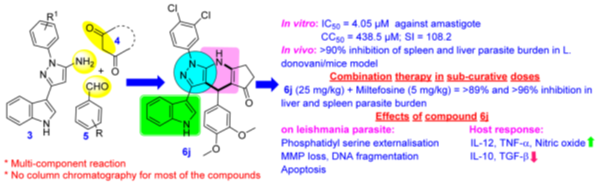
Antileishmanial activity of pyrazolopyridines as an adjunct therapy with miltefosine:
A series of pyrazolopyridine/dihydropyridine analogues has been synthesized and the kwy finding of the study is that the combination treatment with a subcurative dose of miltefosine (5 mg/kg) in BALB/c mice almost completely ameliorated the disease (>97% inhibition) by augmenting nitric oxide generation and shifting the immune response toward Th1. Also, mechanistic investigation revealed that it induced molecular events, such as a loss in mitochondrial membrane potential, externalization of phosphatidylserine, and DNA fragmentation, that ultimately resulted in the programmed cell death of the parasite. These results demonstrated potential of pyrazolopyridine scaffold in treating VL as an adjunct therapy with miltefosine. (J. Med. Chem. 2017, 60, 3, 1041–1059)
-
Antidiabetic activity of carbazole alkaloids isolated from Murraya koenigii leaves:
In this study we have identified koenidine as a metabolically stable antidiabetic compound, when evaluated in a rodent type 2 model (leptin receptor-deficient db/db mice), and showed a considerable reduction in the postprandial blood glucose profile with an improvement in insulin sensitivity. (J. Nat. Prod. 2016, ASAP, DOI: 10.1021/acs.jnatprod.5b00883).
-
Anti-adipogenic compounds from Withania coagulans fruits:
We investigated the effect of coagulin-L on in vitro models of adipocyte differentiation including 3T3-L1 pre-adipocyte, mouse stromal mesenchymal C3H10T1/2 cells and bone marrow derived human mesenchymal stem cells (hMSCs). (Phytomedicine 2014, 21, 406-414).
Development of new methodologies for the synthesis of medicinally active heterocycles:
Synthesis and Antimalarial Activity of 3,3-Spiroanellated 5,6-Disubstituted 1,2,4-Trioxanes:
Novel 3,3-spiroanellated 5-aryl, 6-arylvinyl-substituted 1,2,4-trioxanes have been synthesized and tested for their antimalarial activity against multidrug-resistant Plasmodium yoelii nigeriensis in Swiss mice by oral route. The activity results demonstrated the importance of an aryl moiety at the C-5 position on the 1,2,4-trioxane pharmacophore (ACS Med. Chem. Lett., 2013, 4, 165–169).
Development of new methodologies for the synthesis of medicinally active heterocycles:
Substrate selective synthesis of indole, tetrahydroquinoline and quinoline derivatives via intramolecular addition of hydrazones and imines:
A transition-metal-free, substrate selective methodology for the synthesis of 2,3-diaryl indoles has been developed via the intramolecular addition of hydrazones (bearing no α-H, derived from 2-aminobenzophenones and phenylhydrazines) to in situ generated imine intermediates. However, the hydrazones derived from 2-aminoacetophenones (bearing α-H) produced the corresponding 4-hydrazono-tetrahydroquinolines in a substrate selective manner under the optimized reaction conditions at room temperature. (Org. Chem. Front., 2018, 5, 1170-1175)
PIDA-mediated tandem approach for the synthesis of Benzisoxazole and Benzisothiazole Derivatives:
A practical, mild, and efficient protocol for the synthesis of aryldiazenylisoxazoloarenes from the reaction of 2-amino-N′-arylarylhydrazides has been developed. Additionally, we also demonstrated the synthesis of aryldiazenylbenzisothiazoles in one pot by using a suitable thiation agent (Lawesson’s reagent). (J. Org. Chem. 2015, 80, 12410−12419).
Copper-catalyzed highly efficient oxidative amidation of aldehydes with 2-aminopyridines in an aqueous micellar system:
An eco-friendly and mild protocol has been developed for the synthesis of N-( pyridine-2-yl) amides from aldehydes with 2-aminopyridines by using Cu(OTf)2 as a catalyst and I2 as an oxidant using a micellar system in water. (Green Chem. 2015, 17, 3728-3732).
Transition-Metal-Free C 3 Arylation of Quinoline-4-ones, coumarins and 2-pyridone with Arylhydrazines:
A transition-metal-free C-3-arylation of quinolin-4-ones coumarins and 2-pyridone in the presence of base has been achieved by using arylhydrazines as aryl radical source and air as oxidant. (J. Org. Chem. 2015, 80, 5369−5376; RSC Adv. 2016, 6, 109-118).