Role of SIX3 Homeodomain in the disease Holoprosencephaly and its interactions with Rhodopsin
Holoprosencephaly (HPE) is a severe brain malformation, which results from incomplete separation of forebrain during early embryogenesis. Mutational
analysis has identified four different mutations in the homeodomain of SIX3 that are associated with Holoprosencephaly (HPE). SIX3 plays an important role in vertebrate visual system development. SIX3 HD interacts with specific DNA elements in the rhodopsin promoter to stimulate its transcription resulting in increased rhodopsin expression
β-peptides with proteinogenic side-chains as inhibitors for dimerization of transcription factors.
β-peptide foldamers was taken into consideration as inhibitors for protein-protein interaction because they are invulnerable to proteases with favourable pharmacodynamics and can fold into variety of stable secondary structures i.e., 314 helix, 10/12 helix, 12 helix etc., in solution without the need for tertiary interactions and favourable for cell permeability. In our laboratory we are designing and exploring whether these β-peptide foldamers could bind protein surfaces and inhibit protein–protein interactions and if so, how their affinities and specificities would compared with those of natural or miniature proteins. The targeted ligands are synthesized and their interaction with the targeted proteins will be followed by Chemical shift changes in the
1H-15N HSQC experiment.
Protein:Protein interactions followed ny In-Cell NMR:
The vibriocholera, a gram negative bacterium is a causative agent of cholera disease. This toxin belongs to the family of AB5 bacterial toxins. The disease-causing agent is the enzymatic domain CTA1, and it is intriguing about the folding/unfolding patterns of this domain playing a crucial role in signalling pathway and further in association with
human ARF6, which keeps GSα in continuous active stage thus resulting in diarrhea. Our aim is to understand the interaction of CTA1-ARF-6, as the folding pattern of enzymatic domain by using the InCell NMR techniques.
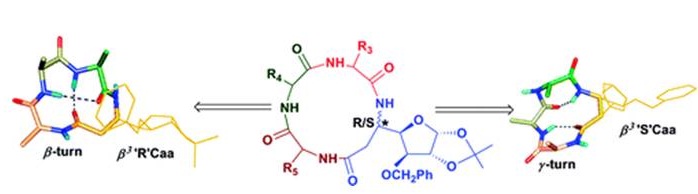
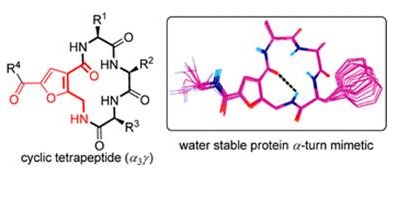
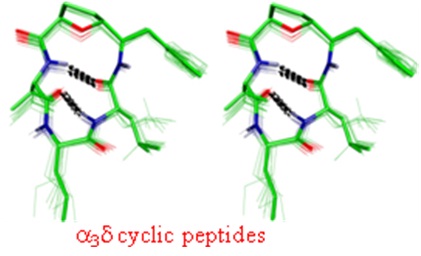
Conformational Studies on Cyclic peptides with α3γ and α3δ architecture. (In collaboration with Dr. D. Koley, CSIR-CDRI and Prof. T. K. Chakraborty, IISC, Bengaluru)
Despite their advantages over small molecules and success of certain blockbuster peptide therapeutics in the market,
the growth of peptide therapeutics have been hampered, because peptides are inherently unstable within the body, they have poor
pharmacological properties, they will rapidly break down into inactive fragments by proteases, and low bio-availability. Normally only one
suitable single conformation is needed for the peptides to interact with their receptors for performing their roles that can be achieved by
restricting their intrinsic conformational flexibility by cyclization and incorporating further conformational constraints in the cycle making
them even more rigid. Cyclic peptides, resistant to both exo- and endoproteases, alleviate the drawbacks of their linear precursors and, in addition,
generate interesting structural and functional features, which bestow them with better therapeutic properties. Our group is involved in exploring
the cyclic peptides, which are predisposed into stable single conformation by incorporation of variety of unnatural aminoacids, along with
proteinogenic α-aminoacids.
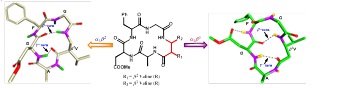
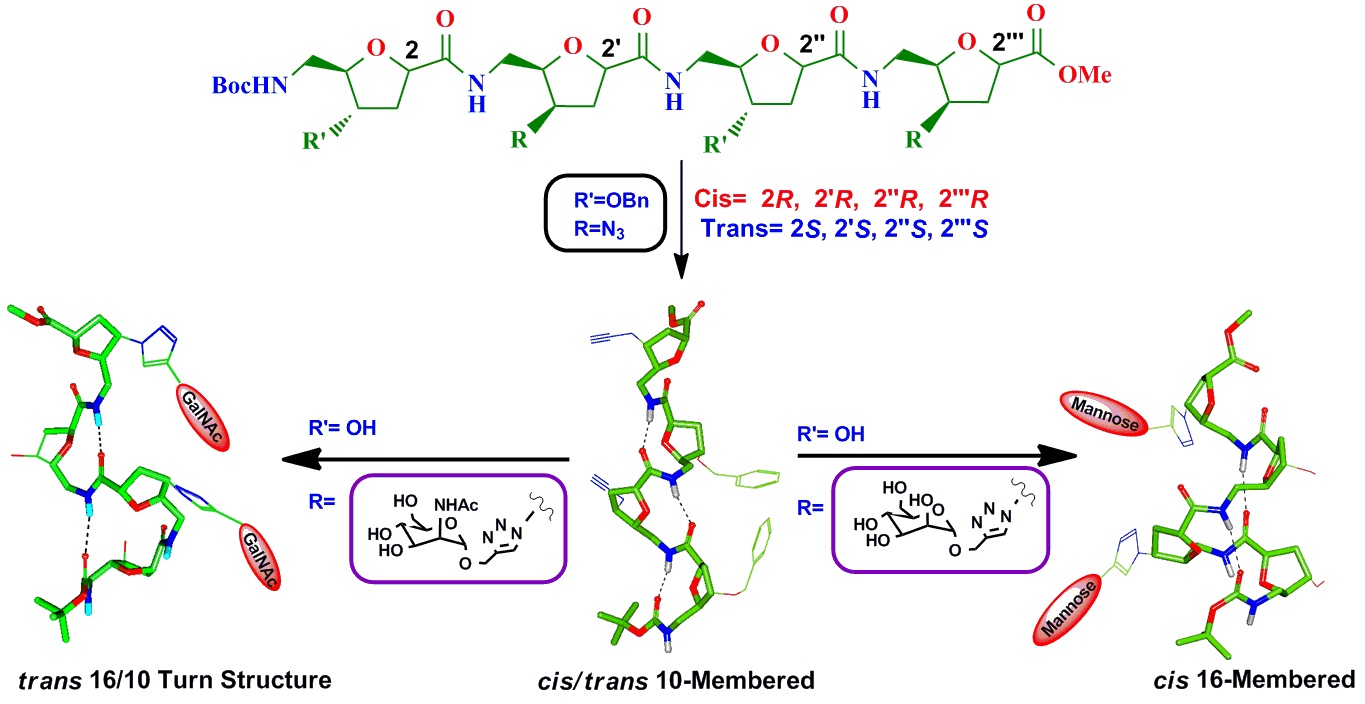
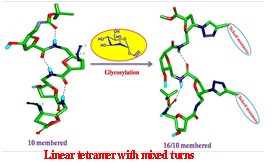
Structure function studies of SAA containing Glycopeptides as inhibitors of Glycosyl Transferase. (In collaboration with Prof. T. K. Chakraborty, IISC, Bengaluru)
GalNAc-transferses are the family of enzymes that control human protein glycosylation during co- and post translational modification.
These enzymes transfer different sugars to cell surface proteins by O-/N- glycosidic linkages with Asn-X-Ser (where X is not proline)
residues via a β-linkage. Glycosyl transferases (GTs) catalyses glycosidic bond formation, either with overall retention or inversion of
anomeric configuration when compared with the stereochemistry in the sugar donor. GTs play important role in many biological process and thus
also involve in many diseases and infections. Due to this they have become now attractive drug targets, and new therapeutic inhibitors can be
designed by detail understanding of their mechanism. Sugar amino acids (SAAs) can be one of the important class of Glycopeptides, which can be
used as building blocks in the search of inhibitors for GTs. Oligomers of SAA’s may overcome the problems associated with oligosaccharide and
peptide libraries such as the susceptibility toward glycosidases due to the altered peptide backbone and their resistance to many proteases due
to their resemblance to carbohydrates.
Structural studies on cyclic and linear analogues of Gramicidin-S with improved biological profiles,
and their detailed structural studies in solution and membrane mimicking media.
(In collaboration with Prof. T. K. Chakraborty, IISC, Bengaluru)
GalNAc-transferses are the family of enzymes that control human protein glycosylation during co- and post translational modification.
These enzymes transfer different sugars to cell surface proteins by O-/N- glycosidic linkages with Asn-X-Ser (where X is not proline)
residues via a β-linkage. Glycosyl transferases (GTs) catalyses glycosidic bond formation, either with overall retention or inversion of
anomeric configuration when compared with the stereochemistry in the sugar donor. GTs play important role in many biological process and thus
also involve in many diseases and infections. Due to this they have become now attractive drug targets, and new therapeutic inhibitors can be
designed by detail understanding of their mechanism. Sugar amino acids (SAAs) can be one of the important class of Glycopeptides, which can be
used as building blocks in the search of inhibitors for GTs. Oligomers of SAA’s may overcome the problems associated with oligosaccharide and
peptide libraries such as the susceptibility toward glycosidases due to the altered peptide backbone and their resistance to many proteases due
to their resemblance to carbohydrates.
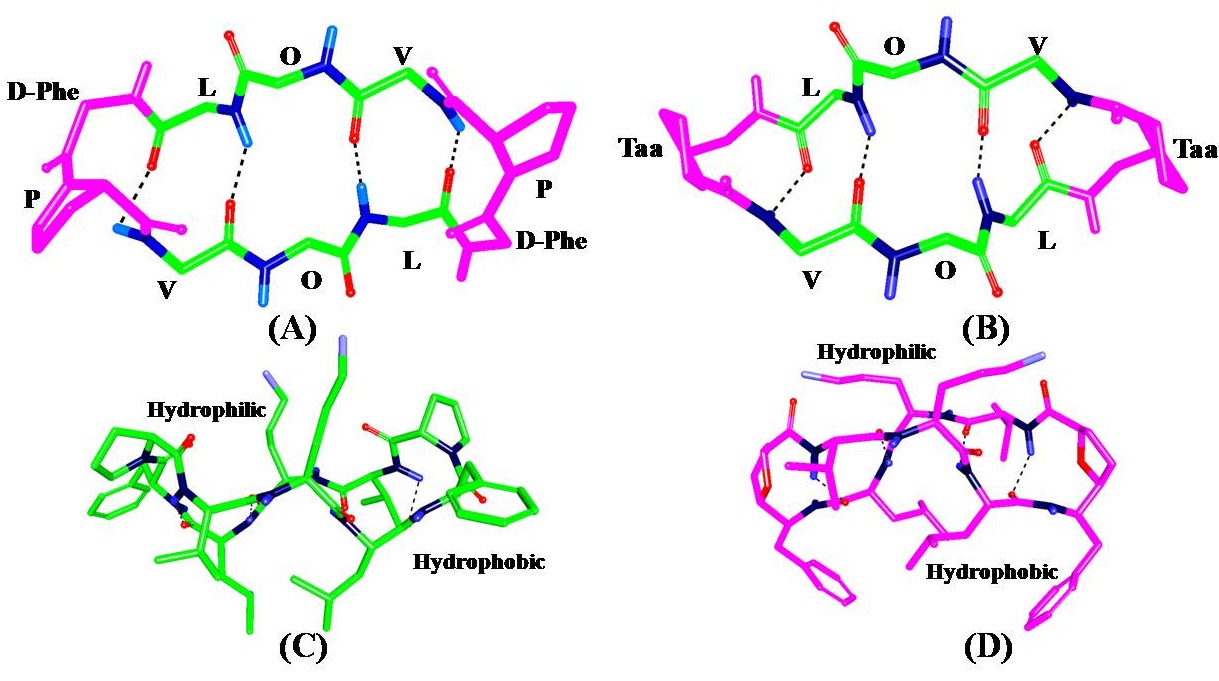
C2-symmetric cyclic decapeptide Gramicidin-S has high potential against both Gram positive and Gram negative bacteria.
It was envisaged that such structural modifications would retain the antimicrobial activity. Our group in collaboration with Prof. Chakraborty’s
group at IISC established the structure function relationship of linear Gramicidin-S analogous peptides by replacing D-Phe and L-Proline residues
with functionalized tetrahydrofuran aminoacid (TAA) isostere carrying a substituent at its C6-position resembling the side chains of D-Phe residues
of Gramicidin. Series of five such linear peptides were designed and synthesized following reported procedure. Our aim is to study the
conformational preferences of these peptides using solution NMR spectroscopy. Further possible mechanism of action of these Gramicidin-S
analogous peptides will be explored in membrane environment using DHPC and DMPC polar lipids
Complete list of publications can be found at
https://scholar.google.co.in/citations?user=mzVeI-4AAAAJ&hl=en