Enantioselective organocatalytic reactions involving N-acyliminium ions and Total synthesis of bioactive natural products
N-acyliminium ions are highly reactive electrophiles that react with weak or soft nucleophiles and offer methods for both intermolecular- and intramolecular carbon-carbon and carbon-heteroatom bond formation. Addition of nucleophiles to N-acyliminium ions furnishes amino derivatives and many other biologically active nitrogen-containing heterocycles. We have developed a bio-inspired asymmetric organocatalytic one pot acyl-Mannich cyclization of hydroxylactam with tethered acetal to afford fused bicyclic alkaloids bearing bridgehead N-atom with excellent optical purities employing co-operative catalysts. Acetal and hydroxylactam were used as pro-nucleophile and pro-electrophile. Both aliphatic and arene substrates were tolerated by the method and cleanly provided pyrrolizidinone, indolizidinone and quinolizidinone derivatives. Total synthesis of (−)-epilupinine, (−)-tashiromine and (−)-trachelanthamidine as the three representative natural products of each sub-type were also achieved to prove the generality of the process.
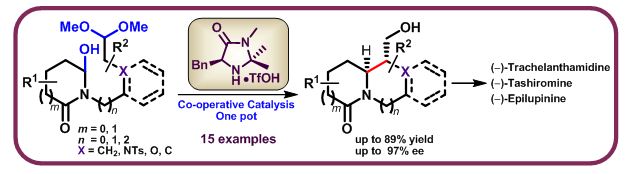
Organocatalytic Asymmetric Mannich Cyclization of Hydroxylactams with Acetals: Total Syntheses of (‒)-Epilupinine, (‒)-
First row transition metal catalyzed C‒H functionalization
Construction of carbon-carbon and carbon-heteroatom bond via direct C‒H bond transformation is very striking strategy in organic synthesis. Considering the easy availability of alkanes in nature, the C‒H bond functionalization strategy could provide practical processes for the synthesis of fine chemical via inexpensive methods. However, selective C‒H bond transformation is the important parameter under forcing conditions. Directing group assisted regioselective C-H functionalization has been developed over the last ten years. We aim to develop regioselective C-H bond transformation via peptide based directing group.
Nickel(II)-mediated regioselective C‒H mono-iodination to arenes and heteroarenes using molecular iodine. Khan, B.; Kant, R.; Koley, D. (communicated)
Design, synthesis and conformational analysis of peptides and peptidomimetics
Protein-protein interactions play key role in many important biochemical events. Synthetic scaffolds having capability to modulate such interactions will have utmost importance in drug discovery process. Although, proteins are macromolecules, only tiny parts having defined secondary structure of the proteins are responsible for these interactions. Therefore, peptide like scaffolds mimicking such important secondary structure could compete with parent proteins during such interactions and hence, have therapeutic potential. The challenge is to design and synthesis these scaffolds with desired secondary structure which will outcompete the parent substrates with very specific manner in the physiological systems.
Furan based Locked Z-Vinylogous -Amino Acid Stabilizing Protein -Turn in Water-Soluble Cyclic 3 Tetrapeptides. Krishna, Y.; Sharma, S.; Ampapathi, R. S.; Koley, D. Org. Lett. 2014, 16, 2084−2087