1. Chemistry of Diazo Compounds
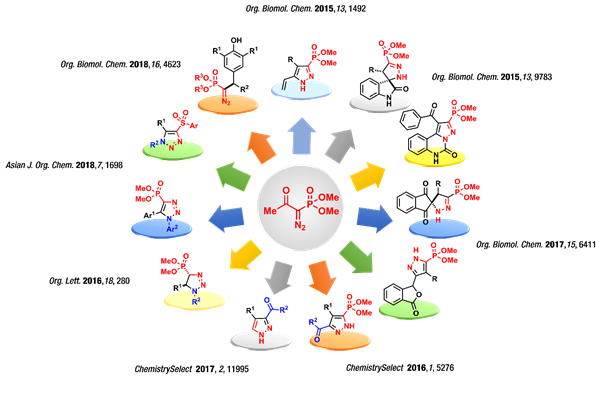
Research in our group is primarily aimed at the development of novel synthetic methods involving diazo compounds for the synthesis of medicinally relevant heterocycles. The Bestmann–Ohira reagent (BOR, dimethyl α-diazo-β-oxopropylphosphonate) is a well-known reagent for the homologation of aldehydes to terminal alkynes under mild conditions, and this reagent is particularly useful for the homologation reactions of delicate substrates. Later, notable reports by Namboothiri and Smietana, as well as recent advances, have shown that the dimethyl (diazomethyl) phosphonate anion (DAMP) generated in situ from BOR could be employed as a versatile 1,3-dipole for the synthesis of phosphonylpyrazoles from numerous electrophilic olefins. Yet, despite the significant progress achieved in this field, most reactions require pre-functionalized olefins to generate the desired pyrazole derivatives. Our research programmes in this area are designed for the direct utilization of commercially available or readily accessible substrates in a domino-multicomponent reaction to create effective and rapid synthetic routes for the construction of synthetically valuable heterocycles.
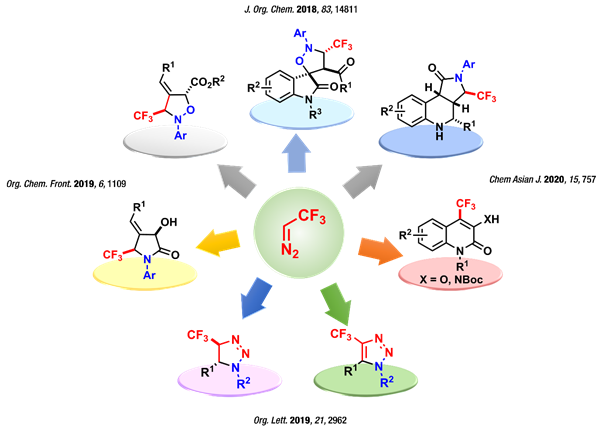
Trifluorodiazoethane, generated by diazotizing trifluoroethyl amine, has been the key reagent to the successful development of several methods for accessing fluorinated chiral and achiral building blocks in a very efficient and atom-economical manner in recent years. The synthetic applications of this unique reagent include metal-catalyzed/mediated synthesis of cyclopropanes using electron-rich olefins, 1,3-dipolar cycloaddition reactions with various electron-deficient olefins and metal-catalyzed C-H insertion reaction reactions. Although substantial progress has been made in the areas mentioned above in the past seven years, consequent to the report on the convenient generation and trapping this gaseous reagent, the unique chemical versatility of this valuable reagent still remains underexplored. In this area, we aim to understand and develop novel reactivity patterns of trifluorodiazoethane by a careful and imaginative selection of substrates, conditions and catalysts.
2. Fluorine Chemistry
The unique properties offered by fluorine when incorporated in a molecule have been the critical factors for the increasingly important role of fluorinated organic compounds in pharmaceuticals, agrochemicals, and material sciences. In consequence, Consequently, the development of effective methods for incorporating fluorine into organic compounds has become an emerging theme of research in the last decades. The most frequently used strategies, particularly in the case of fluorocarbonyl compounds, rely on the metal-catalyzed coupling between aryl halides and appropriately functionalized fluoroalkyl compounds. The use of alpha-fluorocarbanions as nucleophiles for various addition reactions constitutes an efficient alternative approach to access fluoroorganic building blocks. Our research in this area centers at the discovery of novel robust, metal-free conditions for the introduction of vital fluorocarbon subunits into arenes.
3. Medicinal Chemistry
Our research in the area of medicinal chemistry is focused on the design and synthesis of a novel class of aminopropanols as antimalarials. We are also involved in the design and synthesis of small-molecule inhibitors PCSK9.