1. Genetic causes of male infertility
A. Analysis of Y-chromosome micro and partial deletions
In a recent study, we analyzed the AZFc region of the Y-chromosome for complete (b2/b4) and distinct partial deletions (gr/gr, b1/b3, b2/b3). We observed complete AZFc deletions in 0.97% and partial deletions in 6.20% of the cases. Among partial deletions, the frequency of gr/gr deletions was the highest (5.84%). The comparison of partial deletion data between cases and controls suggested a significant association of the gr/gr deletions with infertility (p = 0.0005); however, the other partial deletions did not correlate with infertility. In cohort analysis, men with gr/gr deletions had a relatively poor sperm count (54.20+57.45 million/ml) in comparison to those without deletions (72.49+60.06) (Bansal et al, 2016).
B. Genome-wide screening of male infertility risk factors
We are undertaking genome-wide and candidate gene based SNP panel analysis for identification of new risk factors for male infertility. Exome sequencing and SNP panel based screening of mutations/polymorphisms has identified a number of significant genetic risk factors for male infertility (Gupta et al, 2013). Identification of SNPs that can predict the risk of infertility would pave the way to the development of panels that can be used for infertility risk screening early in life. Early risk prediction would help in family planning in susceptible people.
2. Epigenetic causes of male infertility
Epigenetic modifications characterized by DNA methylation, histone modifications, and chromatin remodeling are important regulators in a number of biological processes, including spermatogenesis. Several genes in the testes are regulated through epigenetic mechanisms, indicating a direct influence of epigenetic mechanisms on the process of spermatogenesis. Aberrations in DNA methylation may affect sperm count and post-fertilization development. We are investigating the whole genome DNA methylation changes in infertile individuals in comparison with fertile controls in order to identify the differentially methylated regions (DMRs) that correlate with human male infertility. One of our recent studies has identified a number of such loci across human genome (unpublished data).
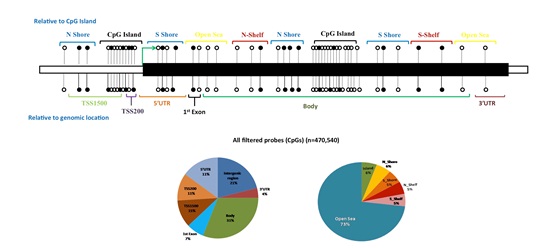 Figure: Whole genome methylation array identified differentially methylated regions that correlate with infertility
Figure: Whole genome methylation array identified differentially methylated regions that correlate with infertility
3. Sperm transcriptome and male infertility
It was believed earlier that spermatozoa have no traces of RNA because of the loss of most of the cytoplasm. Recent studies have revealed the presence of about 3000 different kinds of mRNAs in ejaculated spermatozoa. However, the correlation of transcriptome changes with infertility remains obscure. In a recent study, we identified that about two thousand transcripts are differentially expressed between fertile and infertile sperm. A number of transcripts that participate in a host of cellular processes including reproduction and development were differentially expressed between fertile and infertile individuals. Differences between the comparison groups suggest that sperm RNA has strong potential of acting as marker for fertility evaluation (Bansal et al, 2015).
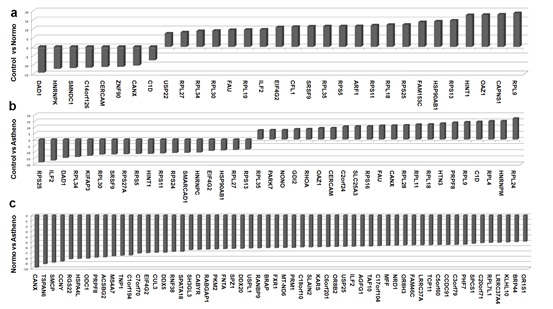 Figure: Differentially expressed transcript between fertile and infertility sperm
Figure: Differentially expressed transcript between fertile and infertility sperm
4. Treatment of male infertility
The Ayurvedic medicinal system claims a number of plants to possess pro-male fertility, aphrodisiac and adaptogenic properties. In a recent study, we demonstrated spermatogenic restorative efficacy of Mucuna pruriens (MP) and its major constituent L-DOPA (LD). In an ethinyl estradiol (EE) based model of spermatogenic loss, we observed efficient and quick recovery of spermatogenesis in MP and LD groups in comparison to the auto-recovery group. The treatment regulated ROS level, apoptosis, and mitochondrial membrane potential (MMP), recovered the hypothalamic-pituitary-gonadal axis and the number of testicular germ cells, ultimately leading to increased sperm count and motility. This is the first study demonstrating that L-DOPA largely accounts for pro-spermatogenic properties of M. pruriens (Singh et al, 2013).
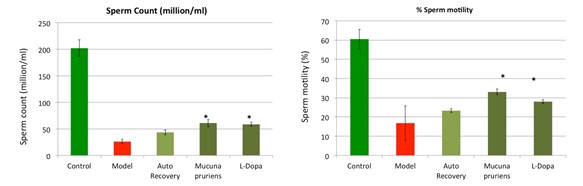 Figure: Mucuna pruriens improves sperm count in an animal model (28 days treatment).
Figure: Mucuna pruriens improves sperm count in an animal model (28 days treatment).