Natural product based drug discovery
Design and Synthesis of ‘nature like molecules’ as potential pharmacological agents:
The complexity and multi-pathogenesis of diseases have prompted drug discovery program to move from traditional ‘‘one protein, one target, and one drug’’ strategy to a rather practical new paradigm in drug research aimed at ‘‘multitarget-directed ligand’’ design strategy. The practical utility of hybridization has been harnessed in designing of therapeutically active agents for the treatment of various ailments. The hybridization approach has resulted into development of multifunctional compounds for the treatment of multifactorial diseases. These multifunctional drugs targeting more than one target of interest have shown to be successful in treating complex disease pathways without any remarkable increase in adverse effects.
1. Novel Chalcone–Thiazole Hybrids as Potent Inhibitors of Drug ResistantStaphylococcus aureus
(Sashidhara KV, et al. ACS Medicinal Chemistry Letters 2015, 6, 809-813.)
2. Benzofuran–Chalcone Hybrids as Potential Multifunctional Agents against Alzheimer’s Disease
(Sashidhara KV, et al. ChemMedChem 2014, 9, 2671-2684.)
3. Discovery of 3-Arylcoumarin-tetracyclic Tacrine Hybrids as Multifunctional Agents against Parkinson’s Disease
(Sashidhara KV, et al. ACS Medicinal Chemistry Letters 2014, 5, 1099-1103.)
4.Discovery of Coumarin−Dihydropyridine Hybrids as Bone Anabolic Agents
(Sashidhara KV, et al. Journal of Medicinal Chemistry 2013, 56, 109-122.)
5.Indole-Based Fibrates as Potential Hypolipidemic and Antiobesity Agents
(Sashidhara KV, et al. Journal of Medicinal Chemistry 2012, 55, 2769-2779.)
Histological findings of the livers of rats supplemented with or without 3f and 3l. In the HFD group, macrovesicular steatosis is present in the centrilobular area (H&E, 200×), whereas steatosis (H&E, 200×) decreases in the 3f, 3l, and fenofibrate supplemented HFD-fed rat group
Isolation and characterization of bioactive natural products by bioactivity directed fractionation and its development as potential leads.
Cytotoxic cycloartane triterpene and rare isomeric bisclerodane diterpenes from the leaves of
Polyalthia longifoliavar. pendula
(Sashidhara KV et al., Bioorganic & Medicinal Chemistry Letters, 2010, 20, 5767-5771)
Two new compounds named Longitriol (1) and Longimide A (2) together with previously known Longimide B (3) were isolated from the leaves of Polyalthia longifolia var. pendula. Compound 1 was tested in vitro against four human cancer cell lines and found to be most active against cervical carcinoma cell lines with IC50(10.03 µg/ml).
Discovery of a new class of HMG-CoA reductase inhibitor from Polyalthia longifolia as potential lipid lowering agent
(Sashidhara KV et al., European Journal of Medicinal Chemistry, 2011, 46, 5206-5211)
Bioassay guided fractionation of the ethanolic extract of Polyalthia longifolia var. pendula, led to the discovery of the clerodane diterpene, 16a-hydroxycleroda-3, 13 (14) Z-dien-15, 16-olide (1), as a new structural class of HMG-CoA reductase inhibitor. Importantly, the in vivo effects of 1 corroborated well with its molecular docking analysis and also with its hamster plasma pharmacokinetics
Galactolipids as a new class of antifilarial agents from Bauhinia racemosa
(Sashidhara KV et al., European Journal of Medicinal Chemistry, 2012, 50, 230-235)
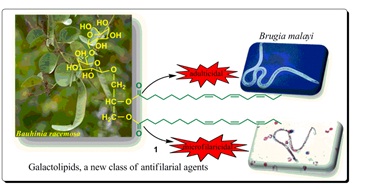
Bioassay guided fractionation of ethanolic extract of the leaves of Bauhinia racemosa led to the isolation of galactolipid and catechin class of the compounds (1-7) from the most active n-butanol fraction (F4). Among the active galactolipids, 1 emerged as the lead molecule which was active on both forms of lymphatic filarial parasite, Brugia malayi. It was found to be better than the standard drug ivermectin and diethylcarbamazine (DEC) in terms of dose and efficacy.
Isolation and identification of β-hematin inhibitors from Flacourtia indica as promising antiplasmodial agents
(Sashidhara KV et al., European Journal of Medicinal Chemistry, 2013, 60, 497-502)
A phenylpropanoid catechin 5 isolated from Flacourtia indica exhibited significant antiplasmodial activity against a chloroquine-resistant (K1) strain. It also inhibited β-hematin formation and showed protection against H2O2-mediated hemin degradation.
The marine sponge metabolite mycothiazole: A novel prototype mitochondrial complex I inhibitor (Morgan et al., Bioorganic and Medicinal Chemistry, 2010, 18, 5988-5994)
The Aignopsanes, a New Class of Sesquiterpenes from Selected Chemotypes of the Sponge Cacospongia mycofijiensis (Tyler et al., Organic Letter, 2009, 11, 1975-1978)
Development of efficient protocols for the synthesis of diverse libraries of biologically relevant scaffolds
In general, the conventional literature approaches for the synthesis of biologically important scaffolds involve the use of multistep reaction sequences. These are typically associated with low yields, high cost, and entail tedious isolation of the resulting products thereby their approach seldom becomes commercially viable. The use of multicomponent reactions (MCRs) has become a modern approach to synthesize such novel compounds of biological significance. The MCR strategy, where three or more simple and flexible molecules are brought together to rapidly introduce structural complexity and diversity, offers significant advantages over conventional linear-type syntheses. Moreover diversity oriented synthesis (DOS) is a important area of research in synthetic organic chemistry to address the demand posed by chemical biology programs for a huge number of small molecules. DOS has many advantages including accessible complexity of molecules, consecutive reaction patterns, high reaction rate, efficiency, and minimal environmental impact.
Integrating the strategies of DOS into the realm of MCRs can be visualized as an elegant synthetic approach to achieve diverse molecular skeletons. In connection with our continuing studies on the development of one pot MCRs to synthesize biologically important heterocyclic scaffolds, we have created skeletal diversity and complexity by integrating the DOS strategy with MCR.
1. Molecular iodine catalysed one-pot synthesis of chromeno[4,3-b]quinolin-6-ones under microwave irradiation
(Sashidhara KV, et al. Green Chemistry 2015, 17, 3766-3770.)
2. An Efficient One-Pot Access to 6,6a-Dihydroisoindolo[2,1-a]quinazoline-5,11-diones and 5-Phenylisoindolo[2,1-a]quinazolin-11(6aH)-ones
(Sashidhara KV, et al. Synlett 2013, 24, 105-113.)
3. A facile and efficient Bi(III) catalyzed synthesis of 1,1-dihydroperoxides and 1,2,4,5-tetraoxanes
(Sashidhara KV, et al. Tetrahedron Letters 2012, 53, 4880-4884.)
4. A Simple and Efficient Access to New Functionalized 4-Phenacylideneflavenes
(Sashidhara KV, et al. Advanced Synthesis & Catalysis 2012, 354, 1129-1140.)
5. Synthesis of 3,6-epoxy[1,5]dioxocines from 2-hydroxyaromatic benzaldehydes
(Sashidhara KV, et al. Tetrahedron Letters 2011, 52, 5659-5663.)