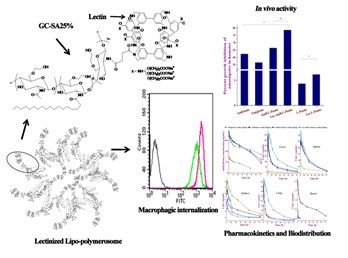
In the area of leishmaniasis major emphasis has been given development of Amphotericin B bearing formulations i.e.
lipo-polymerosome, which was found to have low toxicity that showed synergistic efficacy in case of leishmaniasis
and further explored targeting potential of Lectin functionalized lipo-polymerosome bearing AmB and found that it
has improved pharmacokinetic and pharmacodynamic profile than commercial formulation Fungizone
(Bioconjugate Chem. 25 (6), pp 1091–1102 (2014).
We have also developed oil templated nanocapsules bearing doxorubicin for macrophage targeting through
Phosphatidylserine ligand and established that the ligand can provide a new insight for efficient drug delivery
to specialized macrophages through “eat me” signal (J. Antimicrob. Chemother. 2012; IF 5.068). The chemotherapeutic
potential of alginate coated nanocapsule loaded with doxorubicin was also explored against visceral leishmaniasis in
comparison with nano-emulsions containing doxorubicin [Br. J Pharmacol 2014; IF 4.84; Biomacromolecules 13;16(4):1073-87 (2015)].
We have also observed synergistic enhancement of parasiticidal activity of amphotericin B using copaiba oil in
nano-emulsified carrier for oral delivery: an approach for non-toxic chemotherapy.
The in vitro antileishmanial activity of CopNEC-AmB was significantly higher than that of the free drug as
Cop synergistically enhanced the antileishmanial effect of AmB by causing drastic changes in the morphology of Leishmania
parasite and rupturing its plasma membrane. The CopNEC-AmB showed significantly less haemolytic toxicity and cytotoxicity
and did not change the histopathology of kidney tissues as compared with AmB alone. We have also identified that sulfated
derivatives of a common sugar (4-SO4GalNAc) could be used as a high-performance targeting ligands to target tissue-resident
macrophages especially Leishmania-infected macrophages, suggesting an alternative strategy for the treatment of leishmaniasis.
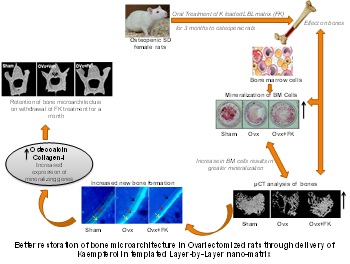
In the area of osteoporosis we have developed Layer-by-layer technology bearing kaempferol which has
impacted on product development by enhancing bioavailability of kaempferol by an order of magnitude
with and enhanced osteogenic efficacy. Single oral dose of kaempferol loaded LbL nanomatrix formulation
increased bioavailability significantly compared to unformulated kaempferol. Three months of formulated
kaempferol administration to osteopenic rats increased plasma and bone marrow Kaempferol levels by 2.8- and 1.75-fold,
respectively, compared to free Kaempferol
Nanomedicine 8(5) 757-771(2013); Eur. J. Pharm. Biopharm. 82 (2012) 508-517
Formulated Kaempferol increased bone marrow osteoprogenitor cells, osteogenic genes in femur, bone formation rate,
and improved trabecular micro-architecture. Withdrawal of Formulated kaempferol-in OVx rats resulted in the
maintenance of bone micro-architecture up to 30 days, whereas micro-architectural deterioration was readily observed
in OVx rats treated with unformulated kaempferol-within 15 days of withdrawal.
(Nanomedicine 2013; IF 5.824 and Eur. J. Pharm. Biopharm 2012; IF 4.245. Also granted US, European, Australia Patents.
In the area of Malaria we have developed novel combination kit bearing arteether and sulfadoxine for the treatment of
P.falciparum Malaria. In this study, the nano-formulation showed 100% curative effect with nearly 1/4th of curative dose
of α/β-arteether in combination of 1/32th of SP curative dose (1983/DEL/2014).
These formulations have been found to be highly effective against Plasmodium yoelii nigeriensis infected
swiss mice even at the lower dose of 12.5 mg/kg x5 days. Overall the developed formulations are safe and
provide a non-toxic platform for further clinical studies and can be used in artemisinin-based combination therapies (ACTs).
Recently we have developed paclitaxel nanocrystals with improved delivery.
Nanocrystals resulted in much enhanced absorption with 12.6 fold improvement in relative
bioavailability to that of TaxolTM. Concomitantly efficacy data in B16 F10 murine
melanoma model also showed significant reduction in tumor growth with NCs compared to TaxolTM and control
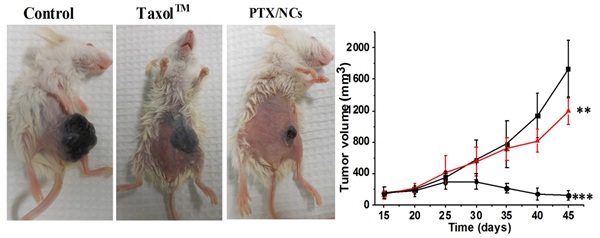
Based on the results it can be suggested that NCs with functional stabilizers can be promising approach
for oral delivery of anticancer drugs which are P-gp substrates (Acta Biomaterialia 2015).