Current Area of Research
• Repurposing of Selected Ongoing Clinical Trial Drugs but nonavailable in India as COVID-19 Therapeutics
In absence of suitable therapeutics for COVID-19, we are working to reposition few known off-patent molecules that are not available in India as possible treatment for SARS-CoV2 in collaboration with company, Ahmedabad through innovation of novel synthetic routes and filing patents on them.
• Atom-economic and cost-effective synthesis of Sunitinib, Nintedanib, Olaparib and Centchroman
Common people having median income cannot afford the treatment arising from using block buster anticancer drugs. The cost effective non-infringing synthetic routes are targeted for (Sunitinib, Nintedanib and Olaparib) since they are having very high selling price as well as high sale but possess low number of Drug Master File (DMF) and suppliers in India. Moreover, can we find out an alternative cost effective and non-infringing synthetic routes for Centchroman for its reposition as anticancer agents?
• Amino acid based inhibitors as dual acting ULK1 and HDAC for autophagy
The designed Amino Acids (AA) based inactivated molecule may be photolyzed using two-colour-two-photon strategies. Thus they become uncaged and simultaneously will be able to interact with the particular receptor followed by exhibiting biological response. Goal is to find AA based cage molecule as double acting role on ULK1 and HDAC to induce autophagy inside the cancerous cell.
• Asymmetric Synthesis of Trisubstituted Methanes (TRSMs) as new bioactives
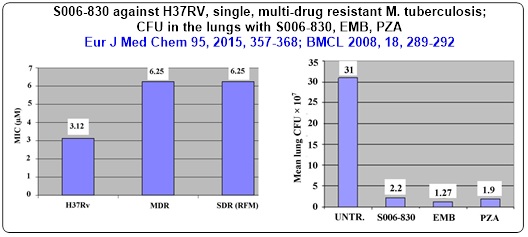
Our group have identified thiophene containing trisubstituted methanes (TRSMs) as a new class of anti-TB molecules after evaluating nearly 300 molecules in this series. One of the lead TRSM showed a statistically significant enhancement in ‘mean survival time’ of Mtb infected mice. It also brought 15-20 fold reduction in the CFU of infected mouse lungs, which was comparable with the efficacy of ethambutol and pyrazinamide. One of the enantiomer S-TRSM (E2) has shown significantly higher MIC and MBC (in vitro as well as ex vivo) than R-TRSM (E1). The goal is to find out new asymmetric cost effective synthesis of individual enantiomers of TRSM for further bioevaluation
• Buckybowl Corannulene derived Amphipathic peptides; Synthesis and Studies on synergistic activities
The objective is to achieve cationic water-soluble amphipathic peptides derived from corannulene and their possible use in biomedical applications to counter ever emerging multi-drug resistance in bacterial infection.
• Q203-Bedaquiline Hybrids as energy metabolism inhibitors for M Tb
Bedaquiline was approved in 2012 for use in treatment of MDR‐TB by the Food and Drug Administration (FDA) and targets the energy metabolism of mycobacteria via inhibition of M TB ATP synthesis in mycobacteria. Q203 is an imidazopyridine amide identified through phenotype high content throughput screening and is currently in phase II clinical trial against MDR and XDR strains of M. tuberculosis. Our target is to synthesize hybrid molecules of bedaquiline and Q203 for potent Anti-Tubercular activity with acceptable side effects
Past Area of Research
• Disruptive Innovative Synthetic Attempts for Drugs like Bedaquiline, Dearylated Lasofoxifene, Centchroman, Meclizine and Cetirizine
In quest for steroidomimetics, employment of chiron Amino Acids to incorporate chiral space towards difficult asymmetric steroids
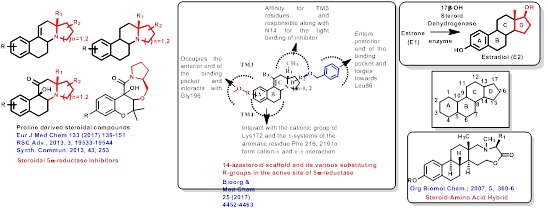
Target and diversity oriented Synthesis of Bioactive natural products from Chiron Amino Acids
Extensive, ingenious harnessing of chiral amino acids (AAs) towards synthesis of potent bioactive molecules
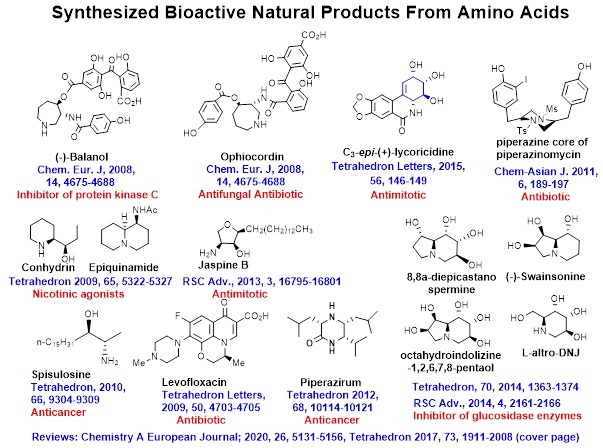
Quest for Steroidomimetics: Amino Acids Derived Chiral Steroidal and non-steroidal architectures as anti breast cancer agents
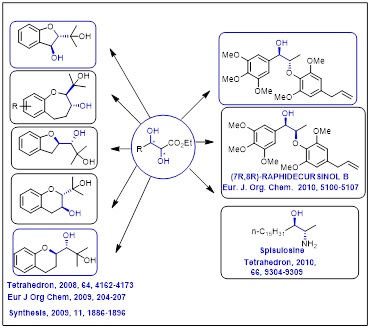
• Syn-2,3-Dihydroxy Esters derived Anticancer molecules
New functionalized synthon to access chiral heterocycles and antimalarial Raphidecursinol B and anticancer Spisulosine
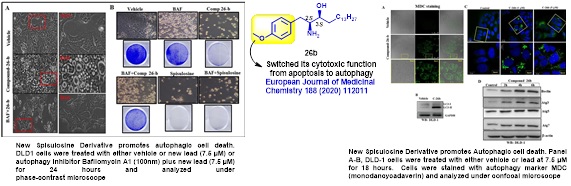
• New Spisulosine Derivative Promotes Robust Autophagic Response to Cancer Cells
Subtle changes in chemical structure of spisulosine completely switched its cytotoxic function from apoptosis to autophagy to address therapy resistance, bringing one of the hallmarks of human cancer
• Chemistry around Chiral Tri and Tetrasubstituted Methanes (TRSMs) as M tb ATP Synthase inhibitors; Asymmetric Synthesis of 9-substituted Xanthenes as constrained TRSMs having anti-TB properties
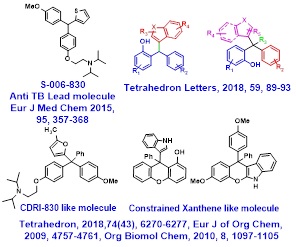
Thiophene containing trisubstituted methanes [TRSMs] as identified lead against Mycobacterium tuberculosis