Association of SNPs with Cancers
Analyses of frequency of single nucleotide polymorphisms on disease or drug-response related genes in diverse populations are important for the
dissection of common diseases. We extensively collected the blood samples from 55 endogamous Indian populations include 32 largeand 23 isolated
populations, representing a large fraction of the people of India. Analyses of data on 405 SNPs from 75 such genes it was observed that high
levels of genetic divergence between groups of populations that cluster largely on the basis of ethnicity and language. Indian populations
overlap with the diversity of HapMap populations, and also contain genetically distinct population groups. We also studied association of
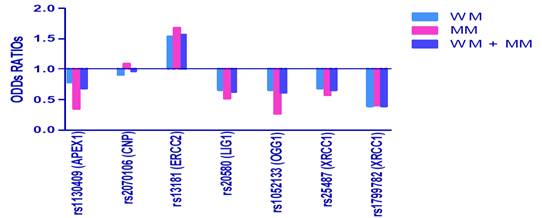
SNPs in the genes of physiological importance in cancers. They are BER,APEX1, XRCC1, FEN1, PCNA, CNP,ERCC2, LIG1, OGG1. BRCA1, BRCA2.
Ammelioration of Toxicity
Cyclophosphamide (CPA) is a widely used chemotherapeutic drug for neoplasias. It is a DNA and protein alkylating agent having a broad spectrum of
activity against a variety of neoplasms including breast cancer. The therapeutic effectiveness of CPA is limited by the high-dose hematopoietic,
renal, and cardiac toxicity that accompanies the systemic distribution of liver-derived activated drug metabolites. The present study examines the
potential of combining resveratrol (RES) with CPA and aims to increase the understanding of the mechanism of cell killing. Interestingly, we
found that RES significantly enhances the caspase-mediated cytotoxic activity of CPA on MCF-7 cells in vitro. RES at 50 μM decreases the IC50
value of CPA from 10 to 5 mM. FACS data reveals CPA or RES alone mediated G0/G1 and S phase arrest, while the combination of these drugs released
both the arrests and results in an increase in the sub G0/G1 peak. Additional analyses indicated the significant up-regulation (P = 0.001) of
tumor suppressor p53 and p53-regulated pro-apoptotic Bax and Fas in MCF-7 cells following CPA treatment in combination with RES, which may
contribute to the enhancement of the antitumor effect of CPA. Furthermore, downregulation of anti-apoptotic Bcl-2 (P = 0.001) was observed
in MCF-7 cells treated with CPA with or without RES when compared to untreated MCF-7. These results suggest the possibility of a new
combination chemotherapeutic regimen leading to improvements in the treatment of breast cancer.
Genotoxicity evaluation
Genotoxicity tests are being performed with new chemical/drug entities to determine and evaluate its health hazards,using standard testing guidelines (e.g. OECD, ICH and Schedule Y). The Tests included are in vitro chromosomal aberrations experiments in human lymphocytes, in vitro mutagenic experiments in Salmonella typhimurium and
Escherichia coli
in vitro and in vivo micronucleus assay, in vivo chromosomal assay in rodents, in vitro Sister chromatid exchange assays in human lymphocyte and in vivo Sister Chromosomal exchange assays in rodents. The laboratory is also involved in delineating detailed molecular mechanism of candidate drugs and NCEs in different target organs using new models and biomarkers.