Project 1: Evolutionary guided, exploration and rational based designing of novel synthetic bio therapeutics with anti-infective activity.
Antimicrobial resistance (AMR) is a major problem in ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas
aeruginosa, and Enterobacter species) group of pathogens, as most of them are part of normal microflora or commensals with an ability to cause infections in immune compromised
state (e.g., surgery, transplantation, cancer, and critically ill patients). ESKAPE pathogens can cause an array of diseases in human due to their unprecedented ability to
inactivate the antimicrobial agents by hydrolysis or modification; alteration or bypassing of drug targets, permeation barriers, and removal of efflux of drugs out of the
cell via efflux pump. Our current understanding on the mode of antibiotic action and the defense mechanism employed by the ESKAPE bacteria indicate that even if we discover
new antibiotics, the bacteria can still resist them by developing biofilms. Hence, more research is required to overcome this critical deficit.
In this regard, host defense peptides (HDPs) have captured the attention of researchers in recent years because of their efficiency as first line of defense against
multi drug resistant pathogens. Cationic host defense peptides (HDPs) are regarded as one of the most promising alternative infectious disease therapies. HDPs, formerly
known as antimicrobial peptides (AMPs), are evolutionary conserved, hydrophobic and amphipathic short (<50 amino acids) cationic molecules with a net charge of +2 to +9.
HDPs are ubiquitous defence biomolecules in nature; they are produced in all organisms ranging from plants and insects to humans, and comprise an integral component of
innate defences against infections. HDPs are short (~50 amino acids), amphipathic, hydrophobic, evolutionary conserved molecules (found in all living organism i.e, from
microbes, plants to humans) with a net cationic charge of +2 to +9. HDPs are expressed either constitutively or during the inflammatory response to infection in the host.
HDPs act by permeabilising bacterial membranes and simultaneously blocking the over activation of the inflammatory molecules by binding to factors like LPS, endotoxins .
Hence, HDPs are regarded as one of the most promising alternative antimicrobial therapies. A critical analysis of the patents and papers published during last 20 years
show that the researchers have taken 1 or 2 microbes as target molecules and completed their study.
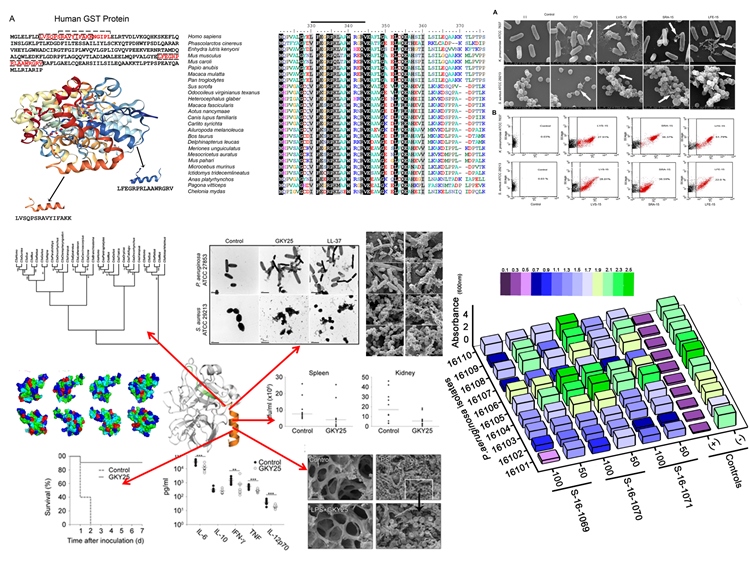
We previously create a large number of peptide variants through structure based evolutionary guided method, sequence scrambling, truncations and systematic
modifications of peptide sequence, and investigated their ability to clear infections (S.aureus and P.aeruginosa) both in vitro and in vivo. We in our search of
antiinfective agents explore evolution conserved epitopes/sequence in the human proteome with potential characteristics for the identification of template. These
templates are subject to screening and further rational based design novel molecules host defence peptides. While hundreds of peptides have been virtually designed
(ie in silico), only a few have been actually synthesized to date. There is still a large pool of broader variety of peptides with excellent predicted antimicrobial
activity. We intendant, from the commercial as well as patent point of view, to create analogs of recently explored novel HDPs for enhanced antimicrobial activity,
antibiofilm, antiquorum sensing, antibiofilm functions.
Project 2: Screening for potential lead molecules for inhibiting various mechanisms involved in microbial pathogenesis mechanism
Undoubted microbes are very dynamic and tough fighters when it comes to survival, they always find a way to protect them from the antimicrobial agents either by making a
armour (biofilm) or destroy (enzymatic) or removing (Efflux) the antimicrobial agents from the cell. Bacterial infections can only be effectively inhibited in pathogens through
the inactivation of all channels or routes that mainly contribute to virulence. Given the basic understanding of microbial defensive mechanism, we focus our attention of
developing the therapeutic agent which can act on different microbes defensive mechanisms or target, so that the pathogens can the controlled for next hundreds of years.
Quorum sensing (QS) is cell communication machinery that is widely used by pathogens to coordinate the expression of genes involved in the production of multiple virulence
factors, biofilm formation, and swarming motility. It is well known fact in infectious diseases biology; pathogenic microbes during infection of a host need to co-ordinate
their virulence in order to escape the immune response of the host and establish a successful infection. To achieve this objective, Pathogenic bacteria developed a unique
way called “biofilm formation”, which is strictly under the QS control. Bacterial biofilms are associated with over 65 percent of all infections. However, no therapeutics
has been approved by the FDA which directly mediates biofilm formation or persistence. Although drugs repurposing seems to be a promising approach to prevent or combat
biofilm infections, it is important to consider that the ideal anti-biofilm candidate would be a drug that is off-patent, safe and works within the maximum recommended
therapeutic dose or safety level.
Recent studies indicate that mimics or analogs compete with the bacteria’s own QS molecules for QS signal receptor binding sites. Further, disruption of QS is a viable
and workable solution for controlling the inter-bacterial spread of virulence factors and fundamental gene-expression, with particular efficacy of biofilm. As biofilm
formation has been proven to have a definite link with quorum sensing, we hypothesize that “inhibiting biofilm development through blocking the quorum sensing or
degrading the signal molecules can increase the efficacy to antibiotics”.
We in the project explore the molecules which can inhibit one or various pathways by which the microbes protect themselves from the lethal punch of antibiotics.
We screen, try to understand the mode of action of various synthetic/natural compounds for antiQS and antibiofilm activity, Efflux pump inhibition.
Project 3: Understanding the interaction of Biotheraputics with innate immune cells during inflammation.
During our work on anti-infective biotheraputics are able to modulate host innate immunity under physiological conditions. Usually, small to moderate damage to the
central nervous system (CNS) evokes protection by microglia. Microglia, resident cell population in the CNS provides the first line of defence against the pathogen
attack on the central nervous system. Even though, microglia and macrophage may be similar in function, there exists a difference between them in morphological anatomy
and cell markers which distinguishingly marks them as separate cell types. Given this observation, we now cannot extrapolate the results obtained from the in vitro
or in
vivo studies using blood derived macrophages to microglia.
In case of intensive acute or chronic activation (in spinal cord injury, optic nerve crush, or stroke) microglia cells become highly neurotoxic and impairs the neuronal
activity. Present evidence collected from literature indicates that under extensive inflammatory conditions, microglia loses its beneficial functions and displays a deleterious
role, further contributing to the spread of damage. Very recently it is observed that the inflammatory responses exacerbated by the endogenous molecules could contribute to
the pathological damage of neurons, neurodegenerative diseases including alzheimer’s disease, Parkinson’s disease and multiple sclerosis. These observations put forward a
question whether the host defence peptides produced by the microglia cell is responsible are useful or harmful to CNS.
Research questions in the line.
1. Whether the host defence peptides (natural/synthetic) interact with the microglia cells and produce pro or anti-inflammatory responses same as macrophages.
2. Whether the response is peptide sequence specific or not, if not, which inflammatory markers do they make in common.
3. Whether the host defence peptides could be counted and investigated as a culprit for any neurodegenerative diseases
like alzheimer’s disease, Parkinson’s disease and multiple sclerosis.
Project 4: Identification and use of novel microbial proteolytic enzymes as drugs to cure auto immune disease and post-transplantation complications
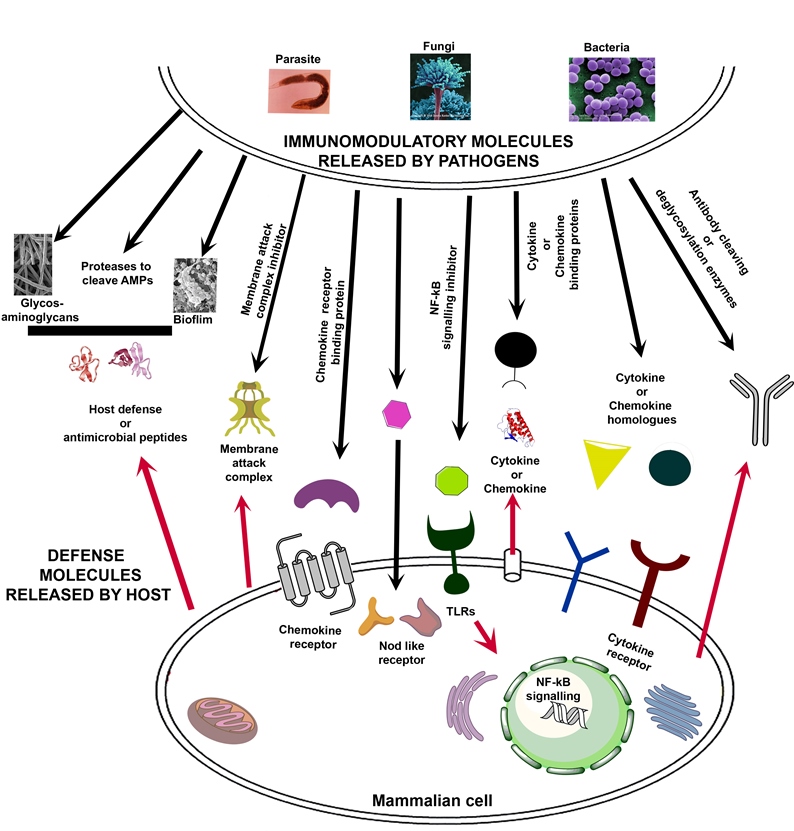
The key to understanding how pathogens cause disease lies in elucidating interaction between pathogen and its host. When a pathogen enters human or animal it first job is
to protect itself from the lethal punch of the host immune system as well establish itself. Most pathogenic microbes produce virulence factors, which either specifically cause
disease or influence the ability of the organism to colonize or persist within the host. For example, Staphylococcus aureus uses hyaluronidase, protease, coagulase, lipases
and enterotoxins as virulence factors where as Streptococcus pyogenes uses M protein, streptokinase, streptodornase, hyaluronidase, spenceronic, dorsettonic, and streptolysins.
It is interesting to note that no two organisms use identical set of virulence factor for their establishment of disease. Even though the molecules used might be different,
most of them belong to proteins class in general, as they use proteins/enzymes as defense mechanism to fight back against the host immune system and establish themselves in
the host.
Human pathogens have developed a wide variety of proteases /virulence factors that destroy various host defense molecules such as antibodies, antimicrobial
peptides, cytokines, or immune cell receptors, before these components can attack the pathogen. These interactions might be of importance for the host parasite
interplay and the development of disease. Proteases are enzymes that account for 1-5% of genomes of infectious organisms. Of specific interest are enzymatic
activities that are targeted towards components of the host immune system. It has been shown by various researchers that Pseudomonas aeurginosa, Group A
streptococcus, Prevotella intermedia, Prevotella nigrescens etc produces a wide variety of powerful proteases which are extremely specific and precise in
their mode of action against immune system. Collin et al have shown that various enzymes from Group A streptococcus have different immunomodulatory functions.
Potempea et.al has shown that Porphyromonas gingivalis produces gingipains, which degrade various immune molecules like IL-6, TNF-α, etc. However there are
still hundreds of other molecules to be discovered from the wealth of microbes.
The lab line of work will be to screen for novel proteases from human pathogens, including Acinetobacter and Burkholderia for use as novel therapeutics.
Once identified, these enzymes will be expressed in large quantities in the bacterial or fungal system, purified, screened for efficiency under in vitro conditions
and studied in autoimmune or inflammation animal model whether they can cure autoimmune diseases and post-transplantation immune-related problems.
The research field pathogen-derived immunomodulatory (IM) molecules are rapidly developing, and several IM’s have been identified as potential immune therapeutics.
Discovery of new immune diseases conditions (i.e., transplant or autoimmune disorders, inflammatory) has drawn the attention of researchers towards microbial enzymes,
especially those that are pathogen-associated, as novel drug targets. Interestingly, the study of microbial enzymes is of dual-use; firstly it helps us to understand
how microbes avoid the detection of the sophisticated and advanced immune system, thus paving the way to develop new drugs which target this vital routine and kill the
bacteria. Secondly, they provide us with molecules by which we can modulate our immune system in the way we want. Proteases with a proven track record of immunomodulatory
properties are attractive alternative candidates to current therapies for autoimmune disorders involving innate and adaptive immune system and organ allograft rejections,
especially in the case of organ transplantation or autoimmune diseases.
Potential researchers interested in joining our team:
Project 1-3: Fresh and trained Master Students or fresh postdocs
Candidates should have a master degree in microbiology or biotechnology or biochemistry or life sciences. It is essential that the successful applicant
should have an absolute passion toward science, a high degree of modesty, and ready to give extra efforts to learn new techniques and methods, the ability to
think critically and logically. A high degree of patience and the ability to accept the failures and move on attitude and humanity is highly appreciated.
Our projects are usually cross-disciplinary in approach involving techniques related to microbiology, Cell biology, Molecular biology, neurobiology, immunology,
and peptide chemistry. In the project (1-3), you will gain experience in Peptide synthesis, handling class II or ESKAPE Pathogens, antimicrobial/antibiofilm assays,
cloning genes for heterologous expression, enzyme/protein purifications to carry out biotransformation reactions, enzyme assays, flow cytometry, HPLC, analysis, Real Time/Quantitative PCR,
acid-urea/native/SDS-PAGE and immunoblotting, western/Slot-blot assay, liposome preparation and leakage assay, Circular dichroism (CD) spectroscopy, animal
infection model, cell cultures, ELISA or bead array assay. By the end of your Ph.D., in addition to the technical skill, we expect you to gain experience
in Statistical analysis, manuscript, and ethical approval permission writing.
Project 4: Potential researchers: Fresh and trained Postdocs
Candidates should have a Ph.D. degree in biotechnology or biochemistry or microbiology with experience in cell culture and microbiology/immunology.
As this is high risk and high rewards area, it is essential that the successful applicant should have an absolute passion toward science, a high degree of
patience and ability to accept the failures and move on attitude, ability to think critically and logically.
This is a cross-disciplinary project involving techniques related to biotherapeutic discovery and drug development. The central core of the project is the
discovery of novel enzymes or molecules used by the pathogen to protect them from the immune system. As this is high risk and high rewards area, we expect the
candidate to be trained in some of the areas like handling Class II or ESKAPE pathogens, immunology techniques, cloning genes for heterologous expression, flow
cytometry, HPLC, protein isolation, purifications, characterisation methods, SDS-PAGE and immunoblotting, western/Slot-blot assay, ELISA, animal infection model,
cell cultures, statistical analysis. The candidate preferably should have the experience in all forms of Scientific writing (manuscript, poster, ethical
approval permission writing).
The project aim is to provide the platform for a successful launch of a scientific career in biotherapeutics area for the successful candidate.