Development of green protocols in the synthesis of bioactive organic molecules: Ionic liquids, microwave, biocatalysis, multi-component reactions (MCRs) and water chemistry as green tools
Thiol–Ene "Click" Reaction Triggered by Neutral Ionic Liquid:
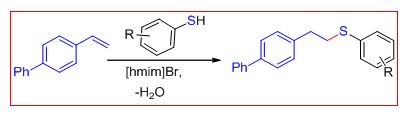
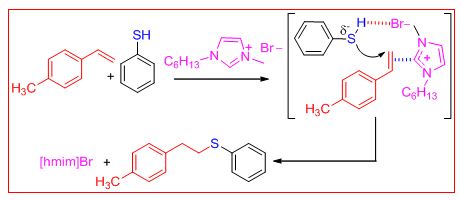
Thiol–ene “click” chemistry has emerged as a powerful strategy to construct carbon–heteroatom (C-S) bonds, which generally results in the formation of two regioisomers.To this end, the neutral ionic liquid [hmim]Br has been explored as a solvent cum catalyst for the synthesis of linear thioethers from activated and inactivated styrene derivatives or secondary benzyl alcohols and thiols without the requirement of using a metal complex, base, or free radical initiator.
Furthermore, detailed mechanistic investigations using 1H NMR spectroscopy and quadrupole time-of-flight electrosprayionization mass spectrometry (Q-TOF ESI-MS) revealed that the “ambiphilic” character of the ionic liquid promotes the nucleophilic addition of thiol to styrene through an anti-Markovnikov pathway. The catalyst recyclability and the extension of the methodology for thiol–yne click chemistry are additional benefits. A competitive study among thiophenol, styrene, and phenyl acetylene revealed that the rate of reaction is in the order of thiol–yne>thiol–ene>dimerization of thiol in [hmim]Br (Sinha et al Angew. Chem. Int. Ed. 2015, 54, 828 –832)
Proposed Mechanism
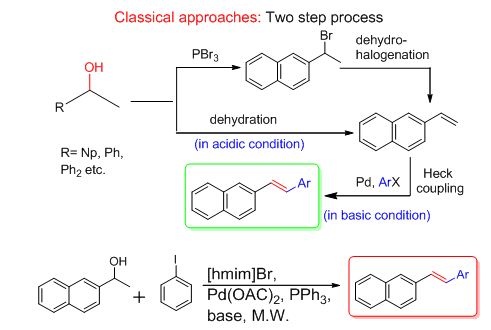
Palladium-Catalyzed Dehydrative Heck Olefination of Secondary Aryl Alcohols in Ionic Liquids:
Our ongoing interest on green chemistry methodology using ionic liquids (ILs) as well as the development of various tandem cross-coupling strategies encouraged us to explore a hitherto unknown approach towards waste-free dehydrative Heck olefination through the concurrent coupling of in situ formed styrenes with aryl halides in ILs where water is the only by-product (Sinha et al Angew. Chem. Int. Ed. 2012, 51, 2636 –2639)
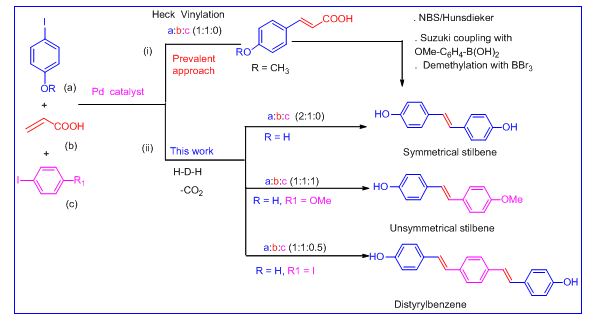
Tandem Heck /Decarboxylation /Heck Strategy: Protecting Group Free Synthesis of Symmetric and Unsymmetric Hydroxylated Stilbenoids:
The palladium-catalyzed tandem Heck/decarboxylation/Heck (HDH) cross-coupling of 4-halophenols with acrylic acid to yield unprecedented symmetric or unsymmetric hydroxylated stilbenoids (C6-C2-C6 unit) or distyrylbenzene (C6-C2-C6-C2-C6 unit) in a protecting-groupfree manner. The method allows tuning of the concentration of the coupling partners, and CO2 is the only by-product with respect to the acrylic acid; conventional coupling of 4-halophenols and acrylic acid are known to provide 4-hydroxy cinnamic acid (C6-C3 unit) (Sinha et al Angew. Chem. Int. Ed. 2012, 51, 12250 –12253)
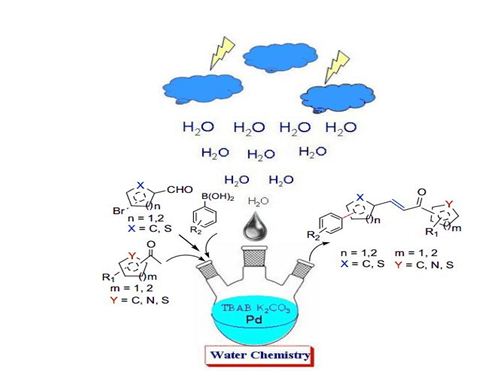
Water Compatible Multicomponent Cascade Suzuki/Heck–Aldol, Suzuki–Aldol–Suzuki, and Aldol–Suzuki–Aldol Reactions:
A water-compatible, highly efficient multicomponent cascade Suzuki– Miyaura– Aldol (S–M–A) reaction of readily available precursors, to form biaryl (hetero)chalcones without the requirement for a ligand or an organic solvent. This further enabled the construction of multiple carbon–carbon bonds in one pot through Suzuki–Miyaura–Aldol–Suzuki–Miyaura (S–M–A–S–M) and Aldol–Suzuki–Miyaura–Aldol (A–S–M–A) reactions in pure water. (Sinha et al Chem. Eur. J. 2013, 19, 14798 – 14803)
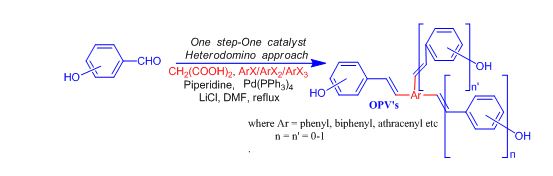
Palladium catalyzed direct olefination of benzaldehydes into oligo(p-phenylenevinylene)s:
A new one pot approach involving Knoevenagel-decarboxylation-Heck sequence has been developed for the direct olefination of benzaldehydes into biologically/industrially important hydroxy functionalized oligo(p-phenylenevinylene)s. In addition, one of the synthesized compounds was also found to show visible and fluorescent fluoride sensing in aqueous/organic medium (Sinha et al Chem. Commun., 2010, 46, 3283)

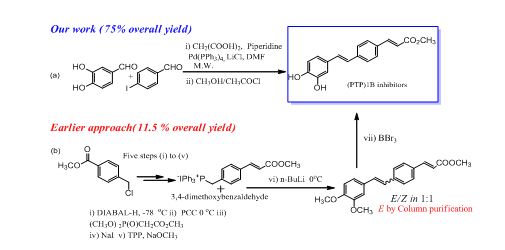
One-pot multi-component synthesis of antidiabetic stilbene cinamoyl hybrids via Pd-catalyzed orthogonal Knoevenagel/Perkin-decarboxylation-Heck/Suzuki sequence:
A one pot double Knoevenagel-decarboxylation-Heck transformation was applied (Sinha et al WO/2010/113005) for the first concise and efficient synthesis of stilbene–cinnamoyl hybrid, a potent protein tyrosine phosphatase (PTP)1B inhibitors (antidiabetic drug candidate) in 75% yield (Sinha et al Chemistry: A Europeon Journal, 2011, 17, 10350 – 10356) as compared to previously reported seven step approach (yield 11.5%). This method also enabled the efficient synthesis of several important hydroxy-functionalized compound classes, such as, cinnamoyl–cinnamic acid hybrids, asymmetric distyrylbenzenes, and biarylstyrenes. Previously reported synthesis require multiple steps and protection/deprotection manipulations.
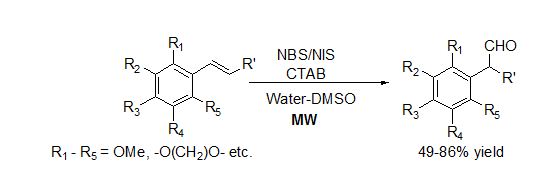
Microwave induced single step synthesis of α-Aryl Aldehydes from arylalkenes in water medium (an intermediate for non-steroidal anti-inflammatory drugs):
A new approach has been developed for one step and metal-free synthesis of biologically and synthetically important α- arylaldehydes from arylalkenes using N-halosuccinimide and phase transfer catalyst in neat water or water-DMSO/Dioxane. (Sinha et al Chem. Commun., 2010, 46, 3283).

Ionic liquid and microwave-assisted decarboxylation of N-heteroaryl and aryl carboxylic acids:
Ionic liquids have been introduced as efficient catalysts cum solvents for metal and quinoline free decarboxylation of diverse N-heteroaryl and aryl carboxylic acids under microwave irradiation (Sinha et al Adv. Synth. Catal., 2008, 350, 2910). The developed methodology offers several inherent advantages like reduction in waste and hazards, recyclability of reagent system, short reaction times besides ease of product recovery
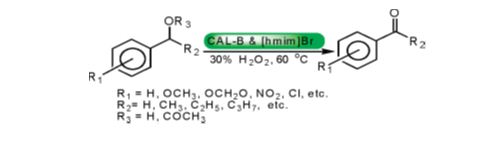
Biocatalytic Promiscuity of Lipase for metal-free activation of H2O2 in Chemoselective Oxidation of Aryl Alcohols/Acetates:
A unique synergistic combination of lipase and ionic liquid [hmim]Br is reported for metal-free H2O2 activation, which is the first example of biocatalytic promiscuity of CAL-B for chemoselective oxidation of aryl alcohols/acetates. The catalytic system exhibits excellent functional group compatibility under neutral conditions besides reusability up to ten cycles thereby making the process economically and environmentally viable (Sinha et al Org. Lett., 2009, 11, 4846).
First Bovine serum albumin promoted synthesis of enones, cinnamic acids and coumarins in ionic liquids:
For the first time, we have uncovered the role of protein impurities in porcine pancreas lipase (PPL), rather than catalytic promiscuity, for olefinic bond formations. Consequently, a novel and highly efficient environment friendly approach involving synergistic catalysis by BSA-[bmim]Br is developed for the synthesis of (E)-α,β-unsaturated compounds including one-pot cascade synthesis of cinnamic acids and coumarins via aldol, Knoevenagel and Knoevenagel-Doebner condensation (Sinha et al Adv. Synth. Catal., 2011, 353, 871-878).