Leishmania actin-network
Actin-network is composed of actin and several actin-binding proteins that regulate its function. Leishmania actin is a non-canonical eukaryotic
actin and possesses distinct biochemical and structural properties (reviewed by Gupta et al., 2015) due to divergence in 6 amino acid stretches.
It is insensitive towards known toxins such as latrunculinB, cytochalasinD and phalloidin and it fails to get incorporated in the mammalian actin
filaments (Sahasrabuddhe et al., 2004). This diverged actin tends to form bundles rather than single filaments and shows high rate of
ATP hydrolysis (Kapoor et al., 2008). Efforts to create gene knockout (null) mutants have not yet been successful indicating essentiality
of this protein in the survival of Leishmania.
a. Coronin
Coronin is an actin-filament binding protein which plays important role in dynamic actions of actin at the cell periphery. Leishmania coronin decorates
filamentous actin in Leishmania and its overexpression induces filament formation (Nayak et al., 2005). Biochemical analysis of the purified Leishmania
coronin indicates that it exists as a stable tetramer through its C-terminal coiled coil domain, which is an important feature for the induction of actin
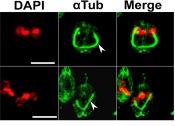
filaments in Leishmania (Srivastava et al., 2015). Genetic ablation of this protein affects dynamics of microtubules at the positive end rendering the
Leishmania cells to become bi-polar. Overall, the defects arise at the terminal stage of cell division (Sahasrabuddhe et al., 2009).
b. ADF/cofilin
ADF/cofilin is an actin-filament depolymerizing factor which regulates actin dynamics within the cells. Leishmania ADF/cofilin homolog, LdCof,
effectively depolymerizes filaments of actins from rabbit muscle or Leishmania. Its actin-filament depolymerizing action is pH insensitive.
Gene knockout (null) mutants and dominant negative mutants of LdCof results in non-motile and stumpy cells with highly reduced flagellum length.
The residual flagellum fails to assemble paraflagellar rod proteins (Tammana et al., 2008; Kumar et al., 2013). Further studies of the mutants
revealed that LdCof is involved in the early stages of the cell division in Leishmania (Tammana et al., 2010). As the motility of Leishmania is
essential for its transmission from one host to another, inactivation of LdCof has a potential to prevent spread of the disease.
c. MyosinXXI
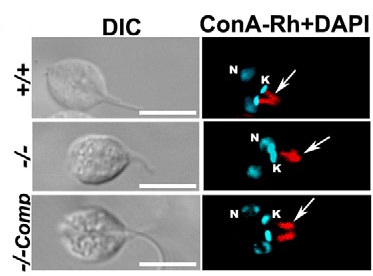
MyosinXXI belongs to a unique class of actin-based motor proteins that are specific to trypanosomatid parasites. The protein in Leishmania localizes mainly at
the base of the flagellum through its C-terminal region (Katta et al., 2009). Genetic ablation of this protein adversely affects flagellum assembly and
intracellular vesicular transport in Leishmania cells (Katta et al., 2010). Null mutants of this protein do not survive indicating it to be a novel drug
target in Leishmania.

d. Twinfilin
Twinfilin is an actin monomer binding proteins that sequesters actin in the cells and thus affects actin drynamics. It is so named because it composes of
two ADF homology domains. In Leishmania, the twinfilin homolog localizes in the nucleolus and redistributes to the elongating mitotic spindles in the
dividing cells. Gene knockout mutants are defective in cell division due to reduction in the pace of DNA synthesis and slowing down the assembly of
mitotic spindle during cell division. Overexpression of Leishmania twinfilin in Leishmania cells results in excessive elongation of mitotic spindle
(Kumar et al., 2016) .
Actin-related proteins
ALeishmania genome contains genes for nine actin-like/actin-related proteins. The closest to actin is called ARP1 while the distant most is ARP9. The
Leishmania ARP1 localizes mainly in the mitochondrion and affects mitochondrial metabolism. A small fraction of total cellular ARP1 also associates with
the flagellum. The gene knockout mutants show significant reduction in mitochondrial membrane potential and depletion of total cellular ATP content. These
mutants also show defects in flagellum length and motility while they are able to assemble paraflagellar rod proteins. The ARP1 gene knockout mutants fail
to proliferate within the mouse peritoneal macrophages (Singh et al., 2014).
Th1 stimulatory proteins and identification of vaccine candidates
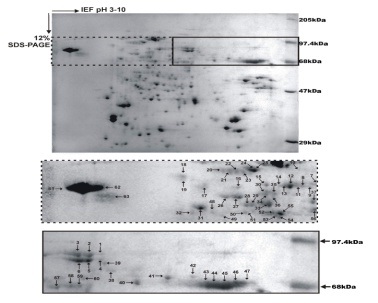
Our group has identified F2 fraction (68 to 97.4 kDa) from soluble proteins of promastigote stage of L. donovani that has shown Th1 type cellular responses
in treated Leishmania-infected patients and hamsters with significant prophylactic efficacy (Garg et al., 2006). Further, sub-fractionation of this
F2-fraction revealed that only four fractions (F2.4–F2.7) belonging to 89.9–97.1 kDa, generated optimal Th1-type cellular responses both individually
as well as in a pooled form (P4-7) (Kumari et al., 2008a; Kumari et al., 2008b). MALDI-TOF/TOF-MS analysis of this pooled protein sub-fraction led to the
identification of 18 proteins as potential vaccine targets (Kumari et al., 2008a; Kumari et al., 2008b). 15 out of 18 proteins were then produced
successfully as the recombinant ones (Baharia et al., 2015; Gupta et al., 2014; Gupta et al., 2012; Khare et al., 2014; Khare et al., 2016; Kushawaha
et al., 2011; Kushawaha et al., 2012a; Kushawaha et al., 2012b) and evaluated simultaneously for their immunogenicity in PBMCs/lymphocytes of treated
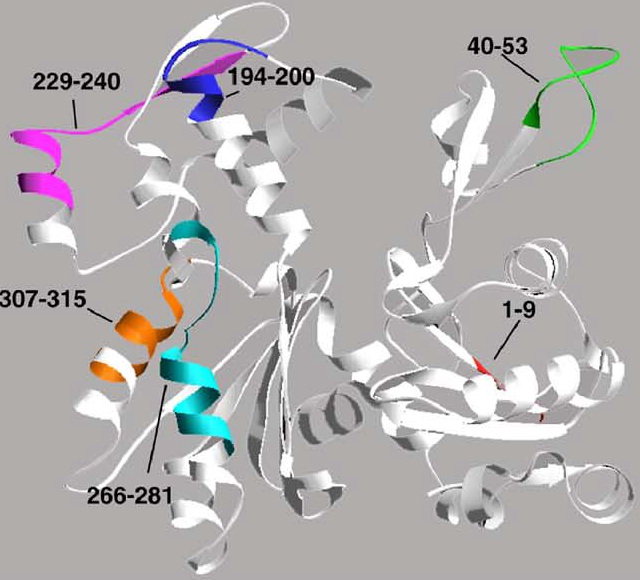
Leishmania patients/hamsters. Six proteins viz. enolase, aldolase, p45, TPI, PDI and elF-2 exhibited good T-cell reactivity in PBMCs of treated VL patients
endorsing the observations made in lymphocytes of treated hamster (Joshi, et al., 2016). However, in order to develop a poly-epitope vaccine,
T-cell epitopes from these six Th1 stimulatory proteins are identified to obtain optimum cellular response.