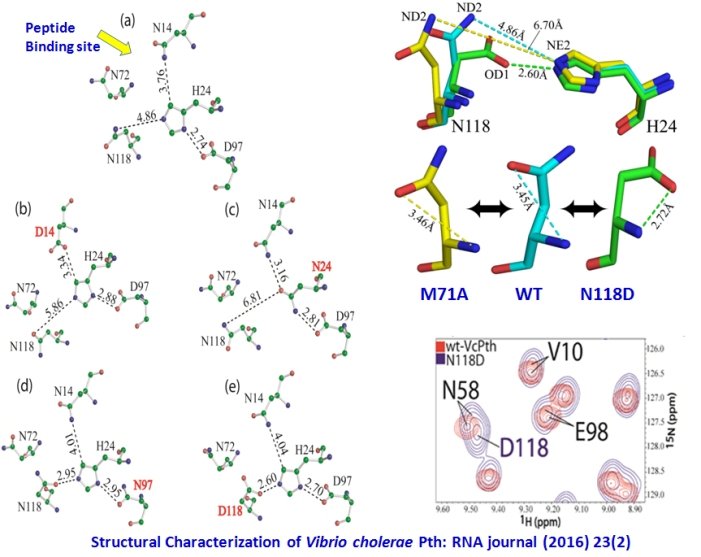
Unraveling the stereochemical and dynamics aspects of the catalytic site of bacterial peptidyl-tRNA hydrolase
Bacterial peptidyl-tRNA hydrolase (Pth; EC 3.1.1.29) is a validated target for the design of antibacterial agents. It hydrolyzes the peptidyl-tRNAs
accumulated in the cytoplasm, and thereby prevents cell death by alleviating tRNA starvation. The activity and selectivity of the Vibrio
cholerae and other Pth proteins depend on the stereochemistry and dynamics of residues H24, D97, N118, and N14. D97-H24 interaction is
critical for activity as it increases the nucleophilicity of H24. N118 and N14 have orthogonallly competing interactions with H24, both of
which reduce the nucleophilicity of H24, and are offset by proper positioning of the peptidyl-tRNA substrate. We have carried out exhaustive
characterization of Pth proteins and mutants of its N14, H24, N72, D97, and N118 residues with X-ray crystallography, NMR spectroscopy, MD
simulations, and DSC measurements. Our studies have provided significantly improved understanding of the hydrogen bonding networks and related
dynamics operating in the structural segments important for the catalysis. These interactions are important to unravel not only mechanistic
understanding of enzyme action but also for design and testing of inhibitors against this protein. NMR assignments of the various mutants
made by us will be very useful for this purpose.
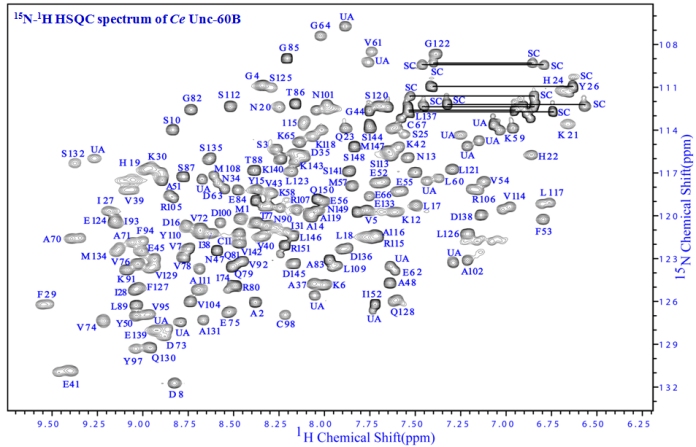
Actin Depolymerizing Factor (ADF) /Cofilin family: Functions and Regulation
ADF binds to G-actin and F-actin and increases the rate of filament polymerization and depolymerization, hence affecting actin dynamics. ADF
increases the turnover of actin filaments by 20-30 fold, which powers actin based motility. ADF is translocated to the regions of high actin
dynamics like leading edge of ruffled membranes, cleavage furrows of dividing cells and neuronal growth cones. Phosphorylation of ADF/cofilin
on an N-terminal serine results in inhibition of actin binding. Activity is also regulated by phosphoinositide binding, pH, the state of the
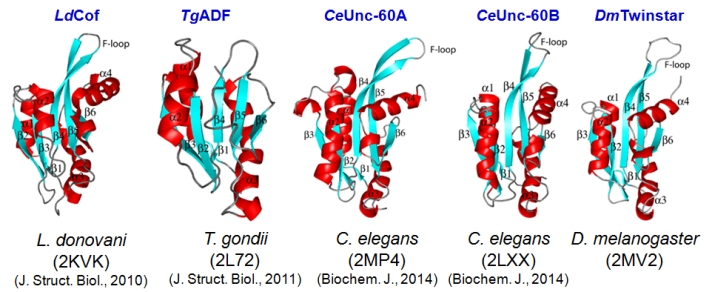
actin-bound nucleotide, and nuclear translocation. We have characterized with NMR spectroscopy the solution structures of ADF/cofilins from
Leishmania donovani, Toxoplasma gondii, Caenorhabditis elegans, and Drosophila melanogaster. Parallely, we have also characterized the
thermodynamics of the interaction of these different ADF/cofilins with rabbit muscle ADP-G-actin by Isothermal Titration Calorimetry. Our
studies provide a fine insight of dynamic regions that help in positioning of the ADF/cofilin protein on the surface of F-actin, which later
leads to severing or depolymerization.
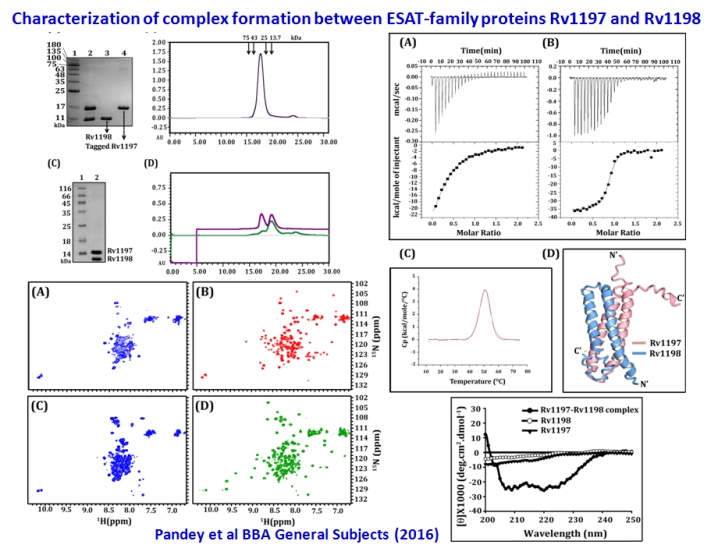
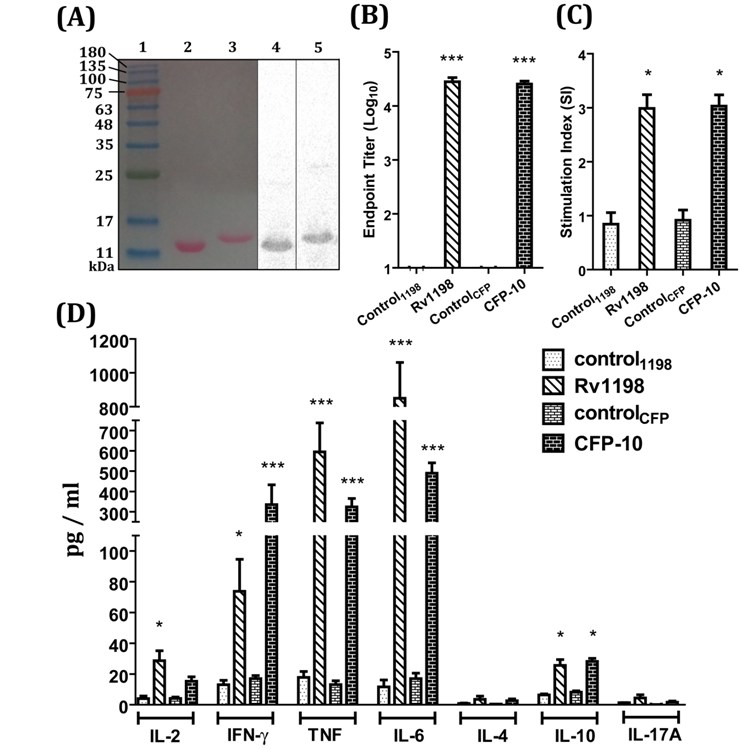
Screening of ESAT-family and other cultural filtrate proteins of Mycobacterium tuberculosis H37Rv for vaccine development
We have extensively characterized by several biophysical methods the complex formation between six different transcriptionally related pairs of proteins of
the ESAT-family. We have covered all the five different Esx systems. We have also characterized Rv3111 (MoaC1), and some of the known antigens like Ag85b
and Rv1813. We have carried out mice immunization with selected antigens and have investigated recall responses of sensitized splenocytes by monitoring
lymphocyte proliferation and induction of cytokines like IFNγ, TNFα, IL-2, IL-4, IL-6, IL-10, and IL-17a. This allows us to pick potential candidates for
combination with other well known antigens for generation of chimeras, which can be developed as subunit vaccine against tuberculosis.